 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| PD-1+VEGF bsAb | Bispecific antibody | Oncology/Cancer | Solid tumor | Phase I | Global (except China) |
| 제품번호 | 종 | 제품 설명 | 구조 | 순도 | 특징 |
|---|---|---|---|---|---|
| VE0-H52H3 | Human | Human VEGF110 Protein, His Tag (MALS verified) |  |

|
|
| VE0-H5212 | Human | Human VEGF110 Protein, premium grade |  |

|
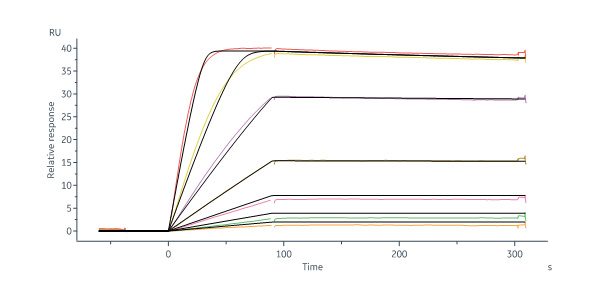
Human VEGFR1/R2 captured on Protein A Chip can bind Human VEGF110 Protein, His Tag (Cat. No. VE0-H52H3) with an affinity constant of 13.8 pM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Ranibizumab biosimilar (Intas Biopharmaceuticals) | Approved | Intas Biopharmaceuticals | Razumab | India | Macular Degeneration | Intas Biopharmaceuticals | 2015-01-01 | Macular Degeneration | Details | |
| Bevacizumab biosimilar (Dr. Reddy's Laboratories) | Approved | Dr Reddy's Laboratories Ltd | Versavo | India | Glioblastoma; Carcinoma, Renal Cell; Peritoneal Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Ovarian Epithelial; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms | Dr Reddy's Laboratories Ltd | 2019-08-19 | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details | |
| Bevacizumab biosimilar (Zydus Cadila) | Approved | Zydus Cadila | Bryxta | India | Carcinoma, Non-Small-Cell Lung | Zydus Cadila | 2017-01-01 | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Peritoneal Neoplasms; Metastatic breast cancer; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Bevacizumab biosimilar(Apotex ) | Approved | Apotex Inc | BAMBEVI | Canada | Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Glioblastoma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Apotex Inc | 2021-09-23 | Carcinoma, Ovarian Epithelial; Glioblastoma; Peritoneal Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Bevacizumab biosimilar (Reliance Life Sciences) | R-TPR-023 | Approved | Reliance Life Sciences | BevaciRel | India | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Glioblastoma; Carcinoma, Renal Cell; Uterine Cervical Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Reliance Life Sciences Pvt Ltd | 2016-06-13 | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (AryoGen Biopharma) | BE1040V | Approved | Aryogen Biopharma | Stivant | Iran | Glioblastoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Uterine Cervical Neoplasms; Metastatic breast cancer; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Aryogen Biopharma | 2019-06-01 | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Peritoneal Neoplasms; Metastatic breast cancer; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Intas Pharmaceuticals) | INTP-24 | Approved | Intas Biopharmaceuticals | Bevatas | India | Carcinoma, Non-Small-Cell Lung; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Uterine Cervical Neoplasms; Glioblastoma; Breast Neoplasms | Intas Biopharmaceuticals | 2017-10-04 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Biocad) | BCD-021 | Approved | Biocad | Avegra | Russian Federation | Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Breast Neoplasms; Fallopian Tube Neoplasms; Peritoneal Neoplasms; Uterine Cervical Neoplasms; Glioblastoma; Carcinoma, Renal Cell | Biocad | 2015-11-25 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Elea) | Approved | Laboratorio Elea Phoenix Sa | Lumiere | Argentina | Macular Degeneration | Laboratorio Elea Phoenix Sa | 2018-04-26 | Macular Degeneration | Details | |
| Ranibizumab biosimilar (Senju) | GBS-007; OT-701; SJP-0133 | Approved | Senju Pharmaceutical Co Ltd | Japan | Macular Degeneration | Senju Pharmaceutical Co Ltd | 2021-09-27 | Macular Degeneration | Details | |
| Bevacizumab biosimilar (Hetero Drugs) | Approved | Hetero Drugs Ltd | Cizumab | India | Colorectal Neoplasms | Hetero Drugs Ltd | 2016-06-27 | Colorectal Neoplasms | Details | |
| Bevacizumab biosimilar (Biocon/Mylan) | MYL-1402O | Approved | Biocon Ltd | KRABEVA, Abevmy, Lextemy | EU | Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Peritoneal Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial | Mylan Ire Healthcare Ltd | 2017-11-27 | Ovarian Neoplasms; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Brain Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Pfizer) | PF-6439535; PF-06439535 | Approved | Pfizer Inc | Zirabev | United States | Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Ovarian Epithelial | Pfizer Inc | 2019-02-14 | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Neovasculgen | PI-VEGF165 | Approved | Human Stem Cells Institute | Neovasculgen | Russian Federation | Peripheral Arterial Disease | Human Stem Cells Institute | 2011-12-07 | Peripheral Arterial Disease; Peripheral Nerve Injuries | Details |
| Bevacizumab biosimilar (Allergan/Amgen) | ABP-215 | Approved | Amgen Inc | Mvasi | United States | Fallopian Tube Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms | Amgen Inc | 2017-09-14 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Qilu Pharma) | QL-1101 | Approved | Qilu Pharmaceutical Co Ltd | 安可达 | Mainland China | Carcinoma, Non-Small-Cell Lung; Colorectal Neoplasms | Qilu Pharmaceutical Co Ltd | 2019-12-06 | Colorectal Neoplasms; Carcinoma, Neuroendocrine; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Celltrion) | CTP-16; CT-16; CT-P16 | Approved | Celltrion Inc | Vegzelma | United States | Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Glioblastoma; Carcinoma, Renal Cell | Celltrion Inc | 2022-08-17 | Ovarian Neoplasms; Carcinoma, Renal Cell; Adenocarcinoma of Lung; Carcinoma, Ovarian Epithelial; Glioblastoma; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Ziv-aflibercept | BAY-865321; AVE-0005 | Approved | Regeneron Pharmaceuticals Inc, Sanofi | Zaltrap | Japan | Colorectal Neoplasms | Sanofi | 2012-08-03 | Lymphoma; Urethral Neoplasms; Breast Neoplasms; Brain Neoplasms; Colorectal Neoplasms; Carcinoma, Mucoepidermoid; Genital Neoplasms, Female; Gliosarcoma; Astrocytoma; Leukemia, Myeloid, Chronic, Atypical, BCR-ABL Negative; Ureteral Neoplasms; Peritoneal Neoplasms; Lung Neoplasms; Prostatic Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Neuroendocrine; Uterine Neoplasms; Endometrial Neoplasms; Glioma; Lymphoma, Non-Hodgkin; Carcinoma, Squamous Cell; Thyroid Neoplasms; Retinal Vein Occlusion; Neoplasm Metastasis; Melanoma; Carcinoma, Non-Small-Cell Lung; Glioblastoma; Leiomyosarcoma; Solid tumours; Multiple Endocrine Neoplasia Type 1; Leukemia; Rectal Neoplasms; Carcinoma; Carcinoma, Renal Cell; Carcinoid Tumor; Colonic Neoplasms; Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Ovarian Neoplasms; Leukemia, Myelomonocytic, Chronic; Myelodysplastic Syndromes; Myeloproliferative Disorders; Carcinoma, Papillary; Carcinoma, Transitional Cell; Small Cell Lung Carcinoma; Ascites; Lung Diseases; Adenoc | Details |
| Bevacizumab biosimilar (Jiangsu Hengrui Medicine) | BP-102 | Approved | Jiangsu Hengrui Medicine Co Ltd | 艾瑞妥 | Mainland China | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Suzhou Suncadia Biopharmaceuticals Co Ltd | 2021-06-22 | Solid tumours; Triple Negative Breast Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Conbercept | FP-3; KH-902 | Approved | Chengdu Kanghong Biotechnologies Co Ltd | 朗沐, Langmu | Mainland China | Macular Degeneration | Chengdu Kanghong Biotechnologies Co Ltd | 2013-11-27 | Macular Edema; Vision Disorders; Diabetic macular oedema; Wet Macular Degeneration; Retinoblastoma; Hemangioma; Uveitis; Corneal Neovascularization; Macular Degeneration; Choroidal Neovascularization; Diabetes Mellitus; Retinal Vein Occlusion | Details |
| Aflibercept | BAT-86-5321; BAY-865321 | Approved | Regeneron Pharmaceuticals Inc, Bayer AG | Eylea | Japan | Retinopathy of Prematurity | Bayer Yakuhin Ltd | 2011-11-18 | Diabetic macular oedema; Corneal Neovascularization; Choroidal Neovascularization; Retinal Vein Occlusion; Carcinoma, Non-Small-Cell Lung; Diabetic Retinopathy; Macular Degeneration; Retinopathy of Prematurity; Neoplasm Metastasis; Lymphoma, Non-Hodgkin; Retinal Degeneration; Eye Diseases; Vitreous Hemorrhage; Diabetes Complications; Colorectal Neoplasms; Retinitis Pigmentosa; Diabetes Mellitus, Type 1; Wet Macular Degeneration; Prostatic Neoplasms; Retinal Diseases; Multiple Myeloma; Myopia, Degenerative; Central Serous Chorioretinopathy; Colonic Neoplasms; Neoplasms; Choroid Diseases; Rectal Neoplasms; Glaucoma, Neovascular; Macular Edema; Diabetes Mellitus, Type 2; Cataract | Details |
| Bevacizumab | G180CU; RO-4876646; RG-435; NSC-704865; G180CL; R-435; G180DL | Approved | Genentech Inc | 安维汀, Avastin | Mainland China | Carcinoma, Hepatocellular | Shanghai Roche Pharmaceuticals Ltd | 2004-02-26 | Solid tumours; Pterygium; HIV Infections; Ependymoma; Ovarian Neoplasms; Kidney Neoplasms; Leiomyosarcoma; Fibrosarcoma; Liver Neoplasms; Epistaxis; Telangiectasia, Hereditary Hemorrhagic; Arteriovenous Malformations; Leukemia; Head and Neck Neoplasms; Leukemia, Myelogenous, Chronic, BCR-ABL Positive; Macular Edema; Abdominal Neoplasms; Telangiectasis; Rectal Neoplasms; Carcinoma, Renal Cell; Meningeal Carcinomatosis; Hemangioblastoma; Esophageal Neoplasms; Anaplasia; Vaginal Neoplasms; Carcinoma; Esthesioneuroblastoma, Olfactory; Carcinoma, Basal Cell; Carcinoid Tumor; Diabetes Mellitus, Type 2; Granuloma, Lethal Midline; Squamous Cell Carcinoma of Head and Neck; Salivary Gland Neoplasms; Nasopharyngeal Neoplasms; Central Serous Chorioretinopathy; Adenocarcinoma of Lung; Glioblastoma; Colonic Neoplasms; Plasmacytoma; Pancreatic Neoplasms; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Myelodysplastic Syndromes; Neurofibromatosis 2; Neoplasms; Blister; Fibrosis; Sepsis; Carcinoma, Verrucous; | Details |
| Ranibizumab | RG-3645; rhu-Fab-VEG; AMD-rhuFab-V2; AMD-Fab; rhuFab-V2; RFB-002; Y-0317; RG-6321 | Approved | Novartis Pharma Ag, Genentech Inc | Lucentis, 诺适得, Susvimo | Japan | Diabetic macular oedema | Novartis Pharma Ag | 2006-06-30 | Vitreous Hemorrhage; Retinoblastoma; Wet Macular Degeneration; Vascular Diseases; Glaucoma; Diabetes Complications; Pseudoxanthoma Elasticum; Retinal Detachment; Retinal Neovascularization; Cardiovascular Diseases; Eye Diseases; Uveitis; Ischemia; Hemangioma; Choroidal Neovascularization; Macular Degeneration; Melanoma; Conjunctival Neoplasms; Retinopathy of Prematurity; Retinal Vein Occlusion; Diabetic Retinopathy; Port-Wine Stain; Corneal Neovascularization; von Hippel-Lindau Disease; Diabetes Mellitus; Myopia; Telangiectasis; Epistaxis; Cataract; Telangiectasia, Hereditary Hemorrhagic; Pterygium; Retinal Telangiectasis; Diabetic Angiopathies; Vitreous Detachment; Iris Diseases; Macular Edema; Vision Disorders; Glaucoma, Neovascular; Choroid Diseases; Optic Neuropathy, Ischemic; Strongyloidiasis; Depression; Neovascularization, Pathologic; Central Serous Chorioretinopathy; Histoplasmosis; Myopia, Degenerative; Retinal Diseases; Angioid Streaks; Pathologic Processes; Glaucoma, Open-Angle; Diabetic macular oe | Details |
| Ranibizumab biosimilar (Xbrane) | Approved | Xbrane Biopharma Ab | EU | Wet Macular Degeneration; Diabetes Complications; Diabetic Retinopathy; Macular Edema | Stada Arzneimittel Ag | 2022-11-09 | Macular Edema; Wet Macular Degeneration; Diabetes Complications; Macular Degeneration; Diabetic Retinopathy | Details | ||
| Faricimab | RG-7716; RO-6867461 | Approved | F. Hoffmann-La Roche Ltd, Genentech Inc | Vabysmo | EU | Wet Macular Degeneration; Diabetes Complications; Macular Edema | Roche Registration Gmbh | 2022-01-28 | Macular Edema; Choroid Diseases; Diabetic macular oedema; Wet Macular Degeneration; Diabetes Complications; Diabetic Retinopathy; Diabetes Mellitus; Retinal Vein Occlusion; Macular Degeneration; Choroidal Neovascularization | Details |
| Bevacizumab biosimilar (mAbixience) | MB02; BEVZ-92; BEVZ92-MB02; AP-01 | Approved | Mabxience Sa | Alymsys | United States | Colorectal Neoplasms | Amneal Pharmaceuticals Llc | 2013-10-25 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Betta/Mabworks) | MIL-60 | Approved | Institute Of Basic Medicine, Chinese Academy Of Medical Sciences, Beijing Mabworks Biotech Co Ltd | 贝安汀 | Mainland China | Glioblastoma; Fallopian Tube Neoplasms; Peritoneal Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Ovarian Epithelial | Betta Pharmaceuticals Co Ltd | 2021-11-24 | Glioblastoma; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular | Details |
| Bevacizumab biosimilar (Bio-Thera Solutions) | BAT-1706 | Approved | Bio-Thera Solutions Ltd | 普贝希 | Mainland China | Carcinoma, Ovarian Epithelial; Fallopian Tube Neoplasms; Glioblastoma; Peritoneal Neoplasms; Uterine Cervical Neoplasms | Bio-Thera Solutions Ltd | 2021-11-17 | Ovarian Neoplasms; Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular | Details |
| Bevacizumab biosimilar (Boan Biopharma) | LY-01008 | Approved | Shandong Boan Biotechnology Co Ltd | 博优诺 | Mainland China | Uterine Cervical Neoplasms; Carcinoma, Ovarian Epithelial | Shandong Boan Biotechnology Co Ltd | 2021-05-07 | Glioblastoma; Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Shanghai Henlius Biotech) | HLX-04; HLX04-O | Approved | Shanghai Henlius Biotech Co Ltd | 汉贝泰 | Mainland China | Glioblastoma; Carcinoma, Hepatocellular | Shanghai Henlius Biotech Co Ltd | 2021-11-30 | Solid tumours; Carcinoma; Rectal Neoplasms; Glioblastoma; Carcinoma, Ovarian Epithelial; Wet Macular Degeneration; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Macular Degeneration; Carcinoma, Hepatocellular | Details |
| Bevacizumab biosimilar (TOT Biopharm) | TAB-008; TOT-102; TAB008; TOT102 | Approved | Tot Biopharm Co Ltd | 朴欣汀, Pusintin | Mainland China | Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Glioblastoma; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms | Tot Biopharm Co Ltd | 2021-12-01 | Carcinoma, Ovarian Epithelial; Glioblastoma; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Ranibizumab biosimilar (Formycon/Bioeq) | FYB-201 | Approved | Formycon, Bioeq Gmbh | CIMERLI, Ranivisio | EU | Macular Edema; Choroidal Neovascularization; Diabetic Retinopathy; Wet Macular Degeneration; Diabetes Complications | Midas Pharma Gmbh | 2022-08-02 | Macular Edema; Wet Macular Degeneration; Diabetic macular oedema; Diabetes Complications; Choroidal Neovascularization; Macular Degeneration; Retinal Vein Occlusion; Diabetic Retinopathy | Details |
| Bevacizumab biosimilar (Samsung Bioepis) | SP-8; SB-8 | Approved | Samsung Bioepis Co Ltd | Aybintio | EU | Uterine Cervical Neoplasms; Carcinoma, Renal Cell; Fallopian Tube Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Samsung Bioepis Nl Bv | 2020-08-19 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Ranibizumab biosimilar (Samsung Bioepis) | SB-11 | Approved | Samsung Bioepis Co Ltd | BYOOVIZ | United States | Macular Degeneration; Macular Edema; Choroidal Neovascularization | Samsung Bioepis Co Ltd | 2021-08-18 | Macular Edema; Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration; Diabetic Retinopathy; Choroidal Neovascularization; Retinal Vein Occlusion | Details |
| Bevacizumab biosimilar (Innovent Biologics) | IBI-305; IBI305; IBI 305; CHS-305 | Approved | Innovent Biologics(Suzhou) Co Ltd | 达攸同, BYVASDA, Bevagen | Indonesia | Triple Negative Breast Neoplasms; Carcinoma, Non-Small-Cell Lung; Ovarian Neoplasms; Uterine Cervical Neoplasms; Colorectal Neoplasms | Innovent Biologics(Suzhou) Co Ltd | 2020-06-17 | Ovarian Neoplasms; Carcinoma, Ovarian Epithelial; Glioblastoma; Triple Negative Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Ranibizumab biosimilar (CJSC Generium) | GNR-067 | Phase 1 Clinical | Cjsc Generium | Macular Degeneration | Details |
| Colorectal cancer vaccine (Immunovo/Pepscan Therapeutics) | Phase 1 Clinical | Pepscan Systems | Colorectal Neoplasms | Details | |
| Ranibizumab biosimilar (Reliance Life Sciences Group) | R-TPR-024 | Phase 3 Clinical | Reliance Life Sciences | Macular Degeneration | Details |
| SCT-501(National Cancer Institute) | SCT-501 | Phase 2 Clinical | National Cancer Institute | Kidney Neoplasms | Details |
| Ziv-aflibercept biosimiliar (Boan Biopharma) | LY-01012; BA-1103 | Phase 1 Clinical | Shandong Boan Biotechnology Co Ltd | Colorectal Neoplasms | Details |
| Bevacizumab biosimilar (Gedeon Richter) | Phase 1 Clinical | Gedeon Richter Plc | Neoplasms | Details | |
| Aflibercept biosimilar (Amgen) | ABP-938 | Phase 2 Clinical | Amgen Inc | Macular Degeneration | Details |
| Bevacizumab biosimilar(Guangdong Dongyangguang) | Phase 1 Clinical | Guangdong Dongyangguang Pharmaceutical Co Ltd | Carcinoma, Non-Small-Cell Lung | Details | |
| Bevacizumab biosimilar (Shanghai Kangdai) | Phase 1 Clinical | Shanghai Kanda Bio-Technology Co Ltd | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Bevacizumab biosimilar (Tanvex BioPharma) | TX-16 | Phase 1 Clinical | Tanvex Biopharma | Colorectal Neoplasms | Details |
| Bevacizumab biosimilar(Guilin Sanjin) | Phase 1 Clinical | Guilin Sanjin Pharmaceutical Co Ltd | Macular Degeneration | Details | |
| Bevacizumab biosimilar(Bioxpress) | BXT-2316 | Clinical | Bioxpress Therapeutics Sa | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Aflibercept biosimilar (Zein Bioteccnology) | Phase 1 Clinical | Zein Bioteccnology Co Ltd | Diabetic macular oedema | Details | |
| CLS-1002 | CLS-1002 | Phase 1 Clinical | Clearside Biomedical Inc | Macular Degeneration | Details |
| Ranibizumab biosimilar (Pfenex) | PF-582 | Pfenex | Details | ||
| Aflibercept biosimilar (Sam Chun Dang Pharm) | SCD-411 | Sam Chun Dang Pharm Co Ltd | Details | ||
| FYB-203 | FYB-203 | Formycon | Details | ||
| Aflibercept biosimilar (Mabwell) | 9-MW-0813; 9MW-0813; 9MW0813; 9-MW0813 | Phase 3 Clinical | Mabwell (Shanghai) Bioscience Co Ltd | Diabetic macular oedema | Details |
| DE-120 | DE-120 | Santen | Details | ||
| AG-13958 | AG-13958; AG-013958 | Pfizer Inc | Details | ||
| Ranibizumab biosimilar (Qilu Pharmaceutical) | BCD-300; QL-1205 | Phase 3 Clinical | Qilu Pharmaceutical Co Ltd, Biocnd | Wet Macular Degeneration; Macular Degeneration | Details |
| Ranibizumab biosimilar (Chong Kun Dang Pharmaceutical) | CKD-701 | Chong Kun Dang Pharmaceutical Corp | Details | ||
| SNN-0029 | sNN-0029 | Newron Pharmaceutical | Details | ||
| Bevacizumab biosimilar (Boehringer Ingelheim) | BI-695502 | C.H. Boehringer Sohn Ag & Co. Kg | Details | ||
| Ranibizumab biosimilar (Lupin) | LUBT-010 | Phase 3 Clinical | Rubin Ltd | Macular Degeneration | Details |
| Bevacizumab biosimilar (North China Pharmaceutical) | MG-021 | Phase 1 Clinical | North China Pharmaceutical Company Ltd | Colorectal Neoplasms; Macular Degeneration; Carcinoma, Non-Small-Cell Lung | Details |
| Dilpacimab | ABT-165; DVD-Ig ABT-165 | Phase 1 Clinical | Abbvie Inc | Solid tumours; Neoplasms | Details |
| ALS-L1023 | ALS-L1023 | Phase 2 Clinical | Angiolab Inc | Non-alcoholic Fatty Liver Disease; Metabolic Syndrome; Macular Degeneration | Details |
| OB-318 | OB-318 | Phase 1 Clinical | Oneness Biotech Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| Bevacizumab biosimilar(Zhejiang Teruisi Pharmaceutical) | TRS-003 | Phase 3 Clinical | Zhejiang Teruisi Pharmaceutical Inc | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Varisacumab | R-84; GNR-011; AT-001-IBCG | Phase 3 Clinical | Peregrine, The University Of Texas Southwestern Medical Center | Glioblastoma; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| IBI-324 | IBI-324 | Phase 1 Clinical | Innovent Biologics (Usa), Inc | Diabetic macular oedema | Details |
| Ranibizumab biosimilar (Shanghai United Cell Biotechnology) | Phase 1 Clinical | Shanghai United Cell Biotechnology Co Ltd | Macular Edema; Macular Degeneration | Details | |
| MP-0250 | MP-0250 | Phase 2 Clinical | Molecular Partners Ag | Neoplasms; Multiple Myeloma; Carcinoma, Non-Small-Cell Lung | Details |
| CT-P42 | CT-P42 | Phase 3 Clinical | Celltrion Inc | Diabetic macular oedema | Details |
| Aflibercept Biosimilar (Alteogen) | ALT-L9 | Phase 3 Clinical | Alteogen Inc, Kissei Pharmaceutical Co Ltd | Macular Degeneration | Details |
| Bevacizumab biosimilar (Genor Biopharma) | GB-222 | Phase 3 Clinical | Genor Biopharma Co Ltd | Brain Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Alphamab/R-Pharm) | RPH-001 | Phase 3 Clinical | R-Pharm | Colorectal Neoplasms | Details |
| Bevacizumab biosimilar (Beijing Science Sun/Beijing Lvzhu) | K-11 | Phase 3 Clinical | Beijing Lvzhu Biological Technology Co Ltd, Beijing Science Sun Pharmaceutical Co Ltd | Liver Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| Bevacizumab biosimilar (Centus Biotherapeutics) | FKB-238 | Phase 3 Clinical | Fujifilm Kyowa Kirin Biologics Co Ltd | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| ASKG-712 | AM-712; ASKG-712 | Phase 1 Clinical | Wet Macular Degeneration; Macular Degeneration | Details | |
| Bevacizumab biosimilar(Laboratorios Sophia) | PRO-169 | Phase 3 Clinical | Laboratorios Sophia Sa De Cv | Diabetic macular oedema | Details |
| Aflibercept Biosimilar(Alvotech Swiss) | AVT-06 | Phase 3 Clinical | Alvotech Swiss Ag | Macular Degeneration | Details |
| Sevacizumab | SIM-BD-0801; EPI-0030; BD-0801; TK-001; APX-003; 9MW0211; 9MW-0211; MW02; MW-02 | Phase 3 Clinical | Epitomics Inc, Apexigen Inc | Ovarian Neoplasms; Solid tumours; Carcinoma, Ovarian Epithelial; Wet Macular Degeneration; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Macular Degeneration | Details |
| VEGFA-targeting Gene Therapy(BDgene) | BD311 | Phase 1 Clinical | Shanghai BDgene Technology Co Ltd | Diabetic macular oedema; Macular Degeneration; Retinal Vein Occlusion | Details |
| Muparfostat sodium | PI-88 | Phase 3 Clinical | Australian National University | Solid tumours; Liver Neoplasms; Neoplasms; Prostatic Neoplasms; Lung Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| Emvododstat | PTC-299 | Phase 3 Clinical | Ptc Therapeutics Inc | Pneumonia; Neoplasms; Neurofibromatosis 2; Coronavirus Disease 2019 (COVID-19); Central Nervous System Neoplasms; Brain Neoplasms; Breast Neoplasms; Coronavirus Infections; Leukemia, Myeloid, Acute; Sarcoma, Kaposi | Details |
| Vanucizumab | RG-7221; RO-5520985; B800Z06O8K (UNII code) | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms; Colorectal Neoplasms | Details |
| Aflibercept biosimilar (CinnaGen) | Phase 3 Clinical | Cinnagen | Macular Degeneration | Details | |
| Bevacizumab biosimilar (Eastern Biotech) | JY-028 | Phase 2 Clinical | Beijing Eastern Biotech Co Ltd | Wet Macular Degeneration; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Macular Degeneration | Details |
| IBI-333 | IBI-333 | Phase 1 Clinical | Innovent Biologics (Usa), Inc | Wet Macular Degeneration; Macular Degeneration | Details |
| Aflibercept biosimilar (Momenta/Mylan) | M-710; MYL-1701P | Phase 3 Clinical | Mylan Nv, Momenta | Diabetic macular oedema | Details |
| TR-009 | NOV-1501; ABL001-ABL Bio; ABL-001-ABL Bio; ES-104; CTX-009 | Phase 3 Clinical | Abl Bio Inc | Biliary Tract Neoplasms; Solid tumours; Rectal Neoplasms; Colonic Neoplasms; Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Bile Duct Neoplasms; Gallbladder Neoplasms | Details |
| BI-836880 | BI-836880 | Phase 2 Clinical | Ablynx Nv | Neoplasms; Wet Macular Degeneration | Details |
| Bevacizumab biosimilar (Hualan Biological Engineering) | WBP-264 | Phase 3 Clinical | Hualan Genetic Engineering Co Ltd | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| ELGN-EYE | ELGN-EYE | Phase 2 Clinical | Elgan Pharma Ltd | Retinopathy of Prematurity | Details |
| Bevacizumab biosimilar (JHL Biotech) | JHL-1149 | Phase 1 Clinical | JHL Biotech | Neoplasms | Details |
| Bevacizumab biosimilar(Shanghai Institute Of Biological Products) | SIBP-04 | Phase 1 Clinical | Shanghai Institute Of Biological Products Co Ltd | Details | |
| Aflibercept Biosimilar (Boan Biopharma/Luye Pharma) | LY-09004; BA-9101; OT-702 | Phase 3 Clinical | Shandong Boan Biotechnology Co Ltd, Luye Pharma Group Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| Aflibercept biosimilar (Samsung Bioepis) | SB-15 | Phase 3 Clinical | Samsung Bioepis Co Ltd | Macular Degeneration | Details |
| Bevacizumab biosimilar (Prestige BioPharma/Hanwha Biologics) | HD-204 | Phase 3 Clinical | Hanwha Biologics | Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Huaota Biopharm) | JS-501; HOT-1010 | Phase 3 Clinical | Shanghai Huaota Biopharmaceutical Co Ltd, Shanghai Junshi Biosciences Co Ltd | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Fudan-Zhangjiang) | Phase 3 Clinical | Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co Ltd | Colorectal Neoplasms; Macular Degeneration; Carcinoma, Non-Small-Cell Lung | Details | |
| Bevacizumab biosimilar(Jiangsu Aosaikang) | ASK-1202; AMD-B; AK-3008; ASK-B1202; ASKB1202 | Phase 3 Clinical | Jiangsu Aosaikang Pharmaceutical Co Ltd | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
This web search service is supported by Google Inc.
