 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 제품번호 | 종 | 제품 설명 | 구조 | 순도 | 특징 |
|---|---|---|---|---|---|
| DK1-C52H7 | Cynomolgus | Cynomolgus DLK1 / FA1 Protein, His Tag |  |
|
|
| DK1-H52H3 | Human | Human DLK1 / FA1 Protein, His Tag (MALS verified) |  |

|
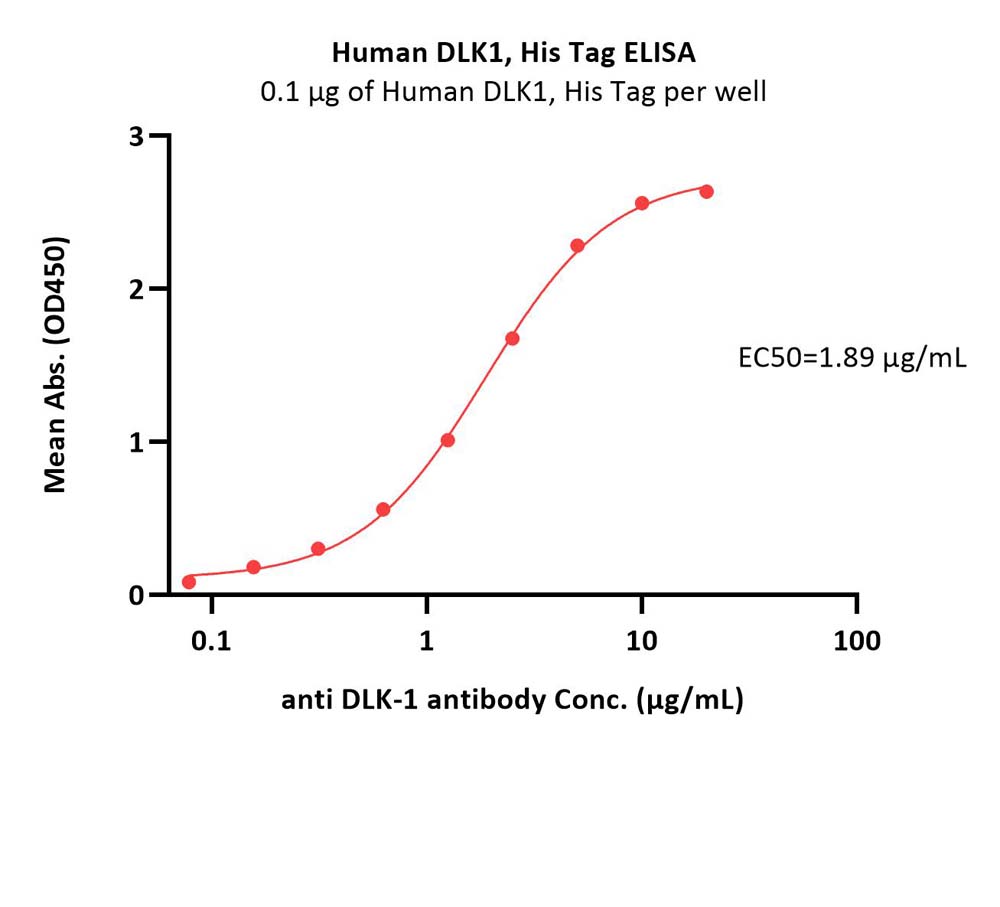
Immobilized Human DLK1, His Tag (Cat. No. DK1-H52H3) at 1 μg/mL (100 μL/well) can bind anti DLK-1 antibody with a linear range of 0.078-5 μg/mL (QC tested).
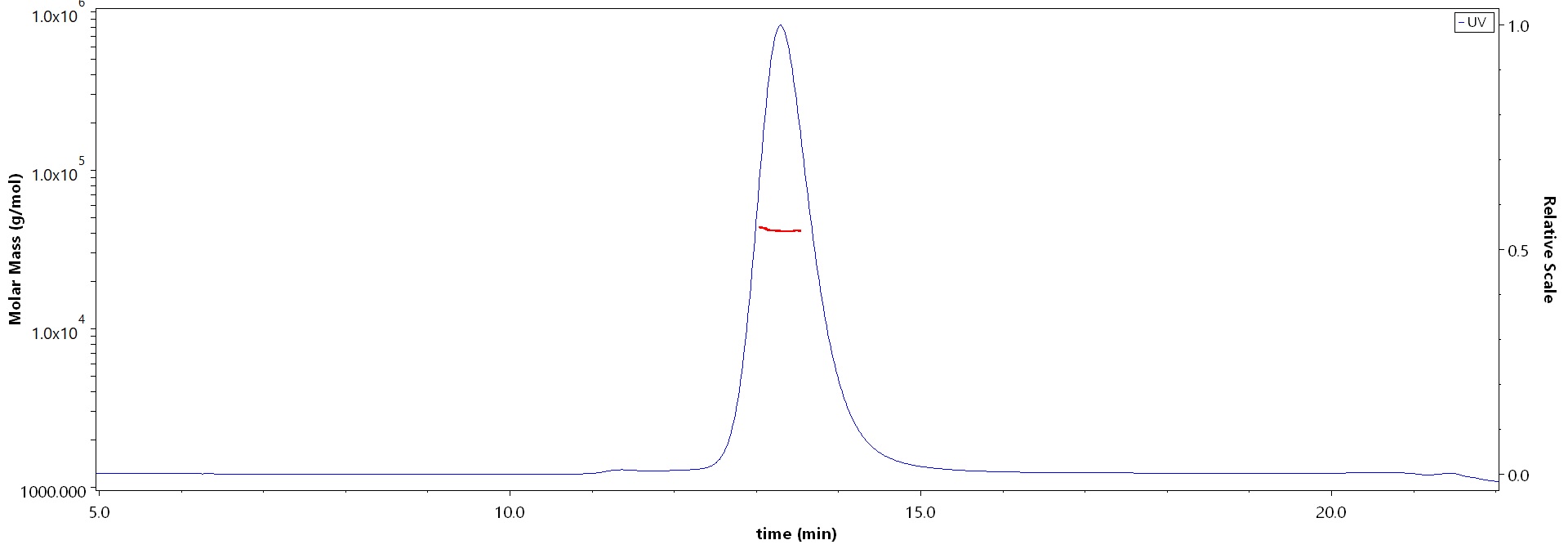
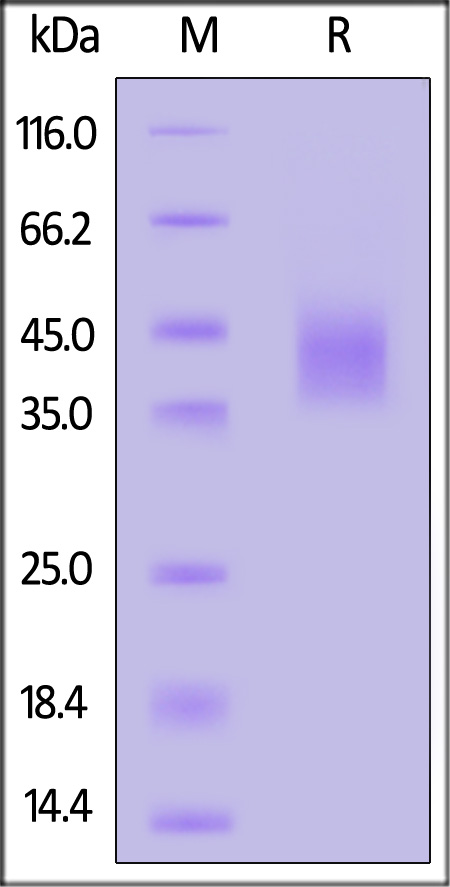
The purity of Human DLK1, His Tag (Cat. No. DK1-H52H3) is more than 90% and the molecular weight of this protein is around 34-51 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Recombinant SARS-CoV-2 vaccine (CHO Cell) (Zhifei Longcom Biopharmaceutical) | ZF2001 | Approved | Anhui Zhifei Longcom Biopharmaceutical Co Ltd, Institute Of Microbiology Of Chinese Academy Of Sciences | 智克威得 | Mainland China | Coronavirus Disease 2019 (COVID-19) | Anhui Zhifei Longcom Biopharmaceutical Co Ltd | 2021-03-01 | Coronavirus Disease 2019 (COVID-19); Coronavirus Infections | Details |
| Recombinant novel coronavirus vaccine (adenovirus type 5 vector) (CanSinoBio) | Approved | Chinese Academy Of Military Medical Sciences, Tianjin Cansino Biotechnology Inc | 克威莎, Convidecia | Mainland China | Coronavirus Disease 2019 (COVID-19) | Tianjin Cansino Biotechnology Inc | 2021-02-25 | Coronavirus Disease 2019 (COVID-19) | Details | |
| Regdanvimab | CT-P59 | Approved | Celltrion Inc | Regkirona | EU | Coronavirus Disease 2019 (COVID-19) | Celltrion Healthcare Hungary Kft | 2021-09-18 | Coronavirus Disease 2019 (COVID-19) | Details |
| Tixagevimab/Cilgavimab | AZD-7442 | Approved | Vanderbilt University Medical Center | Evusheld, 恩适得 | Japan | Coronavirus Disease 2019 (COVID-19) | Astrazeneca Plc | 2022-03-16 | Coronavirus Disease 2019 (COVID-19); Coronavirus Infections; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Sotrovimab | VIR-7831; GSK-4182136 | Approved | Vir Biotechnology Inc | Xevudy | EU | Coronavirus Disease 2019 (COVID-19) | Glaxosmithkline Trading Services Ltd | 2021-08-23 | Solid tumours; Hematologic Neoplasms; Coronavirus Disease 2019 (COVID-19); Lymphoma | Details |
| Amubarvimab/Romlusevimab | BRII-196/BRII-198 | Approved | Tsinghua University, Shenzhen Third People'S Hospital, Brii Biosciences (Beijing) Co Ltd | Mainland China | Coronavirus Disease 2019 (COVID-19) | Tengsheng Huachuang Medical Technology (Beijing) Co Ltd | 2021-12-08 | Coronavirus Disease 2019 (COVID-19); Coronavirus Infections | Details | |
| Casirivimab/imdevimab | REGN-10933+REGN-10987; RG-6413+RG6412; REGEN-COV-2; REGN-COV2; REGN-COV-2; REGEN-COV | Approved | Regeneron Pharmaceuticals Inc, F. Hoffmann-La Roche Ltd | ロナプリーブ, REGEN-COV, RONAPREVE | EU | Coronavirus Disease 2019 (COVID-19) | Roche Registration Gmbh | 2021-07-19 | Coronavirus Disease 2019 (COVID-19); Rejection of organ transplantation; Coronavirus Infections; Chronic Disease | Details |
| Gam-COVID-Vac | Approved | Gamaleya Research Institute Of Epidemiology And Microbiology | Sputnik V | India | Coronavirus Disease 2019 (COVID-19) | Dr Reddy's Laboratories Ltd | 2020-08-11 | Coronavirus Disease 2019 (COVID-19); Coronavirus Infections | Details | |
| Tozinameran | BNT-162b5; BNT162b2SA; BNT162b2; PF-07302048; BNT-161b1; BNT162; Bnt-162b2 | Approved | Biontech Se, Pfizer Inc | Comirnaty | United States | Coronavirus Disease 2019 (COVID-19) | BioNTech Manufacturing GmbH | 2020-12-02 | Infections; Anaphylaxis; Coronavirus Disease 2019 (COVID-19); Neoplasms; Severe Acute Respiratory Syndrome; Coronavirus Infections | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| ABP-300 | ABP-300; MW-05(Mabwell/Abpro) | Phase 3 Clinical | Mabwell (Shanghai) Bioscience Co Ltd, Abpro Corp | Coronavirus Disease 2019 (COVID-19) | Details |
| JMB-2002 | JMB-2002 | Phase 1 Clinical | Coronavirus Disease 2019 (COVID-19) | Details | |
| Casirivimab | RG-6413 | Phase 2 Clinical | F. Hoffmann-La Roche Ltd | Coronavirus Disease 2019 (COVID-19) | Details |
| Romlusevimab | BRII198; BRII-198 | Phase 1 Clinical | Coronavirus Disease 2019 (COVID-19) | Details | |
| Amubarvimab | BRII-196; BRII196 | Phase 1 Clinical | Coronavirus Disease 2019 (COVID-19) | Details | |
| DXP-593 | BGB DXP593; DXP-593 | Phase 2 Clinical | Singlomics Biopharmaceuticals Co Ltd | Coronavirus Disease 2019 (COVID-19) | Details |
| MW-33 | MW-33; 9MW3311; 9-MW3311; 9MW-3311; 9-MW-3311 | Mabwell (Shanghai) Bioscience Co Ltd | Details | ||
| HLX-71 | HLX-71 | Shanghai Henlius Biotech Co Ltd | Details | ||
| BGB-DXP604 | DXP-604 | Phase 2 Clinical | Beigene Ltd, Singlomics Biopharmaceuticals Co Ltd | Coronavirus Disease 2019 (COVID-19) | Details |
| Plutavimab | STI-2020 | Sorrento Therapeutics Inc | Details | ||
| MAD-0004J08 | MAD-0004J08 | Phase 1 Clinical | Fondazione Toscana Life Sciences | Coronavirus Disease 2019 (COVID-19) | Details |
| LY-CovMab | LY-CovMab | Phase 2 Clinical | Luye Pharma Group Ltd | Coronavirus Disease 2019 (COVID-19) | Details |
| JS-026 | JS-026 | Shanghai Junshi Biosciences Co Ltd | Details | ||
| SI-F019 | SI-F019; F-019; SZ-F019 | Sichuan Baili Pharmaceutical Co Ltd | Details | ||
| MTx-COVAB36 | MTx-COVAB36 | Memo Therapeutics AG | Details | ||
| abdavomeran | BNT162b1 | Biontech Se | Details | ||
| GRT-R-912 | GRT-R912; GRT-R-912 | Gritstone Bio Inc | Details | ||
| GRT-R-914 | GRT-R914; GRT-R-914 | Gritstone Bio Inc | Details | ||
| GRT-R-918 | GRT-R918; GRT-R-918 | Gritstone Bio Inc | Details | ||
| TY-027 | TY-027 | Tychan | Details | ||
| IMM-BCP-01 | IMM-BCP-01 | Immunome Inc | Coronavirus Disease 2019 (COVID-19) | Details | |
| F-61 | F-61 | Phase 1 Clinical | Wuhan Institute Of Biological Products Co Ltd | Coronavirus Disease 2019 (COVID-19) | Details |
| COVI-SHIELD | STI-9167; STI-9199 | Phase 2 Clinical | Icahn School Of Medicine At Mount Sinai | Coronavirus Disease 2019 (COVID-19) | Details |
| GSK-4362620A | GSK-4362620A | Phase 3 Clinical | Medicago Inc, Gsk Vaccines Gmbh | Coronavirus Disease 2019 (COVID-19) | Details |
| REGN-15160 | REGN-15160 | Phase 1 Clinical | Regeneron Pharmaceuticals Inc | Coronavirus Disease 2019 (COVID-19) | Details |
| SCB-2020S | SCB-2020S | Phase 1 Clinical | Clover Biopharmaceuticals Aus Pty Ltd | Coronavirus Disease 2019 (COVID-19) | Details |
| Etesevimab | LY-3832479; LY-CoV016; JS-016 | Phase 2 Clinical | Institute Of Microbiology Of Chinese Academy Of Sciences, Shanghai Junshi Biosciences Co Ltd | Coronavirus Disease 2019 (COVID-19); Severe Acute Respiratory Syndrome | Details |
| Ogalvibart/Crexavibart | BMS-986414 + BMS-986413 | Phase 3 Clinical | The Rockefeller University, Bristol Myers Squibb Srlcompany | Coronavirus Disease 2019 (COVID-19); Coronavirus Infections | Details |
| Imdevimab | RG-6412 | Phase 3 Clinical | F. Hoffmann-La Roche Ltd | Coronavirus Disease 2019 (COVID-19) | Details |
| Betuvax-CoV-2 | Phase 2 Clinical | Human Stem Cells Institute, Russia | Coronavirus Disease 2019 (COVID-19) | Details | |
| BAT-2022 | BAT-2022 | Phase 1 Clinical | Bio-Thera Solutions Ltd | Coronavirus Disease 2019 (COVID-19) | Details |
| Lomtegovimab | BI-767551 | Phase 2 Clinical | German Center For Infection Research, University Of Cologne | Coronavirus Disease 2019 (COVID-19) | Details |
| IBIO123 | IBIO-123 | Phase 2 Clinical | Immune Biosolutions Inc | Coronavirus Disease 2019 (COVID-19) | Details |
| FBR-002 | FBR-002 | Phase 2 Clinical | Fabentech | Coronavirus Disease 2019 (COVID-19) | Details |
| CG-SpikeDown | Phase 1 Clinical | Caregen Co Ltd | Details | ||
| AVACC-10 | AVACC-10 | Phase 1 Clinical | Intravacc | Coronavirus Disease 2019 (COVID-19) | Details |
| ADM-03820 | Phase 3 Clinical | Ology Bioservices Inc | Coronavirus Disease 2019 (COVID-19); Severe Acute Respiratory Syndrome | Details | |
| CT-P63 | CT-P-63; CT-P63 | Phase 1 Clinical | Celltrion Inc | Coronavirus Disease 2019 (COVID-19) | Details |
| Upanovimab | HB-27; SCTA-01 | Phase 3 Clinical | SinoCelltech Ltd | Coronavirus Disease 2019 (COVID-19) | Details |
| Recombinant SARS-CoV-2 Vaccine(variant)(Shanghai Zerun) | Phase 2 Clinical | Shanghai Zerun Biotechnology Co Ltd | Coronavirus Disease 2019 (COVID-19) | Details | |
| COR-101 | STE90-C11 | Phase 2 Clinical | Corat Therapeutics Gmbh | Coronavirus Disease 2019 (COVID-19) | Details |
| Ad5-triCoV/Mac | Ad5-triCoV/Mac | Phase 1 Clinical | Canadian Institutes Of Health Research (Cihr) | Coronavirus Disease 2019 (COVID-19) | Details |
| STI-2099 | STI-2099 | Phase 2 Clinical | Sorrento Therapeutics Inc | Coronavirus Disease 2019 (COVID-19) | Details |
| HY-3000 | HY-3000 | Phase 2 Clinical | Hybio Pharmaceutical Co Ltd | Coronavirus Disease 2019 (COVID-19) | Details |
| Bamlanivimab | LY-3819253 | Phase 3 Clinical | AbCellera Biologics Inc | Coronavirus Disease 2019 (COVID-19) | Details |
| Enuzovimab | HFB-3013; HFB30132A | Phase 1 Clinical | HiFiBiO Therapeutics | Coronavirus Disease 2019 (COVID-19) | Details |
| Equine immunoglobulin anti SARS-CoV-2 (Caja Costarricense de Seguro Social) | Phase 2 Clinical | Universidad De Costa Rica | Coronavirus Disease 2019 (COVID-19) | Details | |
| PTX-COVID19-B | Phase 3 Clinical | Providence Therapeutics Holdings Inc | Coronavirus Disease 2019 (COVID-19) | Details | |
| Ogalvibart | BMS-986414 | Phase 1 Clinical | Bristol Myers Squibb Srlcompany, The Rockefeller University | Coronavirus Disease 2019 (COVID-19) | Details |
| Crexavibart | C144-LS; C144LS; BMS-986413 | Phase 1 Clinical | The Rockefeller University, Bristol Myers Squibb Srlcompany | Coronavirus Disease 2019 (COVID-19) | Details |
| Bamlanivimab/Etesevimab | Eli Lilly And Company, Shanghai Junshi Biosciences Co Ltd | Details | |||
| AKS-452-X | AKS-452-X; AKS-452X | Phase 2 Clinical | Akston Biosciences Corp | Coronavirus Disease 2019 (COVID-19) | Details |
| Bebtelovimab | LY-CoV1404 monoclonal antibody; LY-3853113; LY-CoV 1404 mAb; LY-CoV1404 | Phase 2 Clinical | Eli Lilly And Company | Coronavirus Disease 2019 (COVID-19) | Details |
| Reluscovtogene ralaplasmid | INO-4800; INO4800; PGX-9501 | Phase 2 Clinical | Inovio Pharmaceuticals Inc | Coronavirus Disease 2019 (COVID-19); Coronavirus Infections; Hepatitis B | Details |
This web search service is supported by Google Inc.
