 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 제품번호 | 종 | 제품 설명 | 구조 | 순도 | 특징 |
|---|---|---|---|---|---|
| NKA-M52H5 | Mouse | Mouse NKG2A / CD159a Protein, His Tag |  |
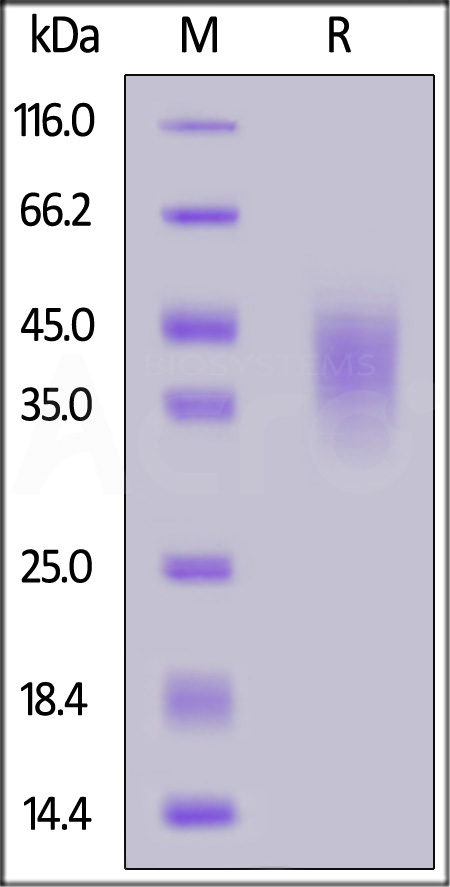
|
|
| NKA-C5245 | Cynomolgus | Cynomolgus NKG2A / CD159a Protein, His Tag |  |
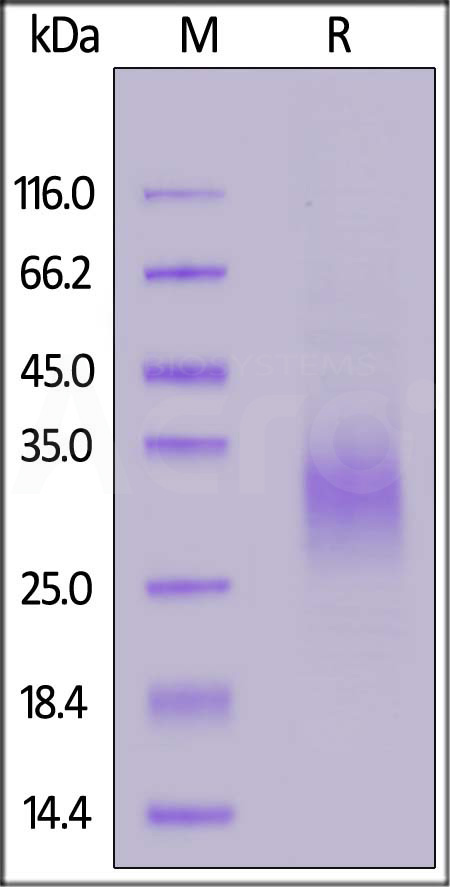
|
|
| NKA-H526c | Human | Human NKG2A / CD159a Protein, Fc Tag |  |

|
|
| NKA-H5244 | Human | Human NKG2A / CD159a Protein, His Tag |  |
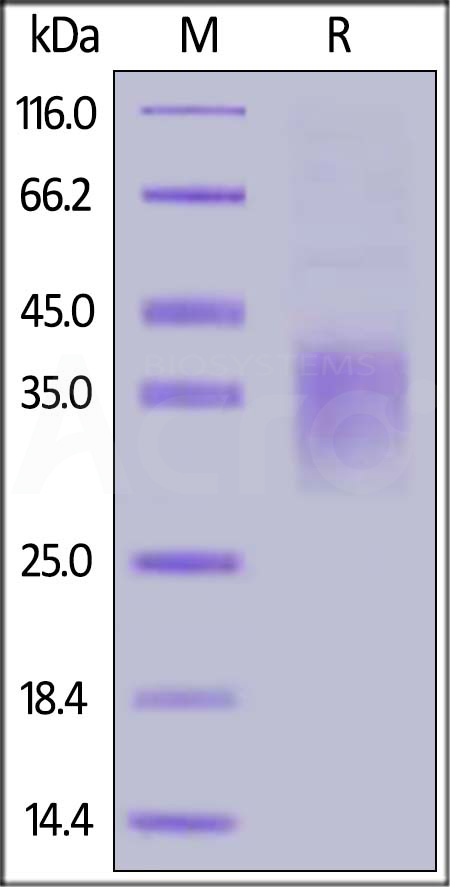
|
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| S-095029 | S-095029 | Phase 1 Clinical | Institut De Recherches Internationales Servier | Solid tumours | Details |
| HY000102 | HY-0102; HY-000102 | Phase 1 Clinical | Shanghai HyaMab Biotech Co Ltd | Solid tumours | Details |
| Monalizumab | NN-8765; IPH-2201; NNC-0141-0000-0100 | Phase 3 Clinical | Novo Nordisk A/S, Innate Pharma | Head and Neck Neoplasms; Hematologic Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Arthritis, Rheumatoid; Breast Neoplasms; Genital Neoplasms, Female; Inflammation; Carcinoma, Squamous Cell; Esophageal adenocarcinoma; Esophageal Squamous Cell Carcinoma; Mouth Neoplasms; Leukemia, Lymphocytic, Chronic, B-Cell; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung | Details |
This web search service is supported by Google Inc.
