 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 제품번호 | 종 | 제품 설명 | 구조 | 순도 | 특징 |
|---|---|---|---|---|---|
| GA3-H5247 | Human | Human Galectin-3 Protein, His Tag |  |
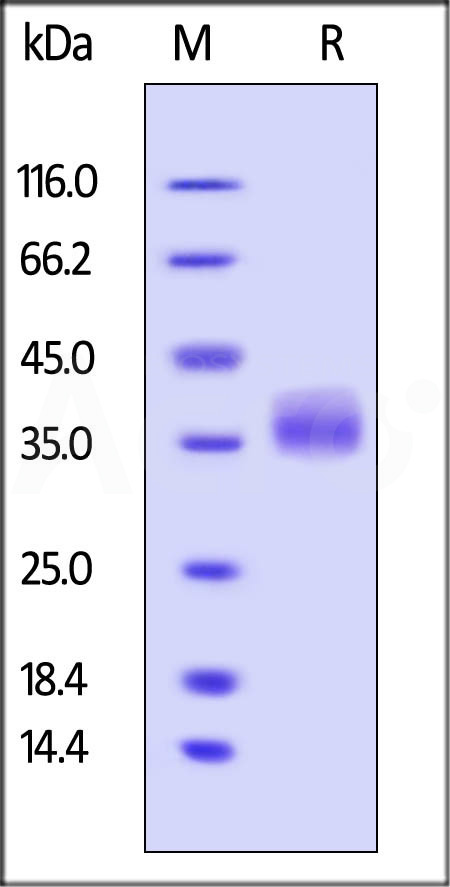
|
|
| GA3-H5129 | Human | Human Galectin-3 Protein, His Tag (MALS verified) |  |

|
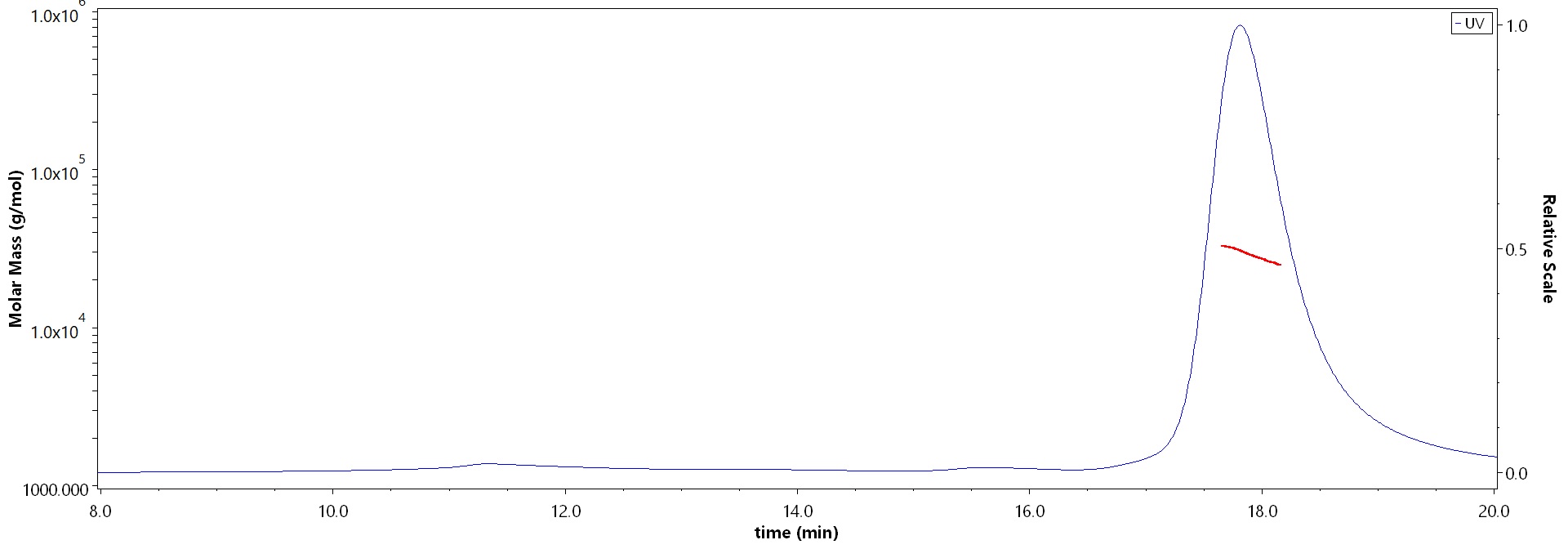
The purity of Human Galectin-3, His Tag (Cat. No. GA3-H5129) is more than 90% and the molecular weight of this protein is around 23-35 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Ocriplasmin | A-01016; THR-409 | Approved | Thrombogenics | Jetrea, Jejetrea | EU | Retinal Diseases | null | 2012-10-17 | Vitreous Detachment; Arterial Occlusive Diseases; Disease Progression; Tissue Adhesions; Stroke; Retinal Diseases; Diabetic macular oedema; Uveitis; Vitreomacular Adhesion; Venous Thrombosis; Eye Diseases; Macular Degeneration; Diabetic Retinopathy; Retinal Vein Occlusion | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Gal-400 | Gal-400 | Phase 1 Clinical | Non-alcoholic Fatty Liver Disease; Liver Cirrhosis | Details | |
| GB-1211 | GB-1211 | Phase 2 Clinical | Galecto Inc | Non-alcoholic Fatty Liver Disease; Hepatic Insufficiency; Carcinoma, Non-Small-Cell Lung | Details |
| Olitigaltin | GB-0139; TD-139 | Phase 2 Clinical | Lund University Foundation, The University Of Edinburgh | Idiopathic Pulmonary Fibrosis; Coronavirus Disease 2019 (COVID-19) | Details |
| Belapectin | GR-MD-02 | Phase 3 Clinical | Galectin Therapeutics Inc | Non-alcoholic Fatty Liver Disease; Squamous Cell Carcinoma of Head and Neck; Fibrosis; Hypertension, Portal; Psoriasis; Hepatic Insufficiency; Esophageal and Gastric Varices; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
This web search service is supported by Google Inc.
